Main Article Content
Abstract
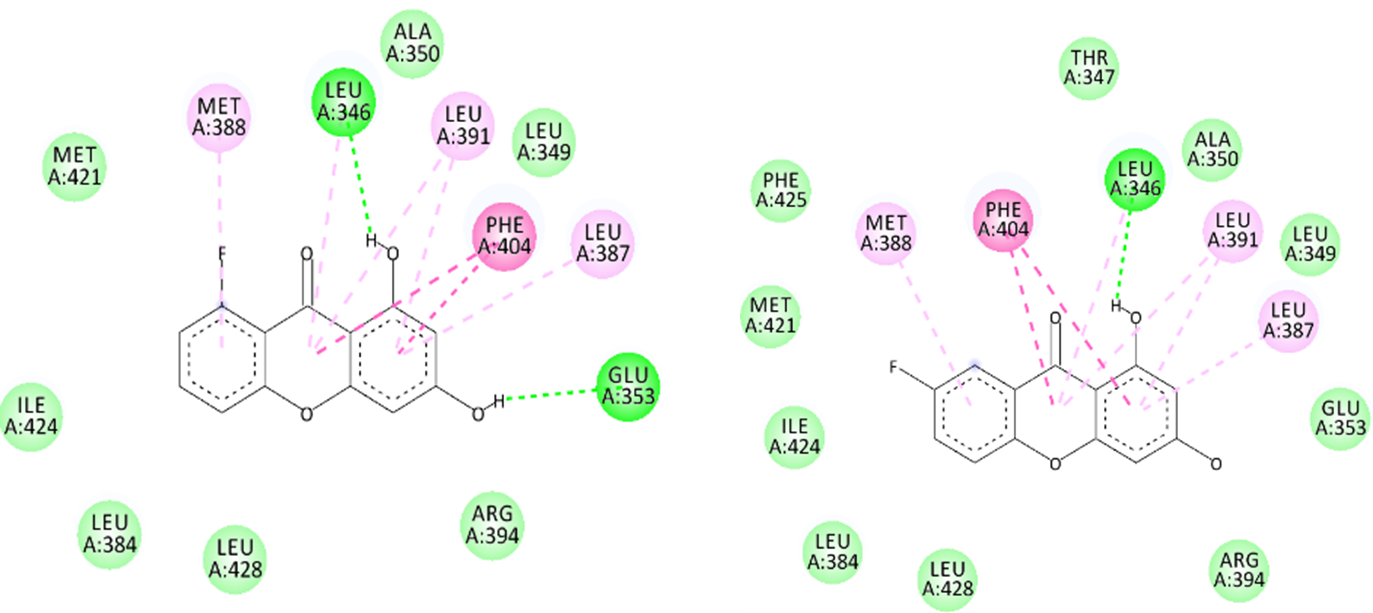
Fluoro-hydroxyxanthone derivatives (X1–X10) were investigated as potential inhibitors of estrogen receptor alpha (ER-α) through molecular docking and in silico ADMET profiling. The docking protocol was validated through a redocking procedure, yielding a Root Mean Square Deviation (RMSD) of 0.81 Å, which confirmed the method's reliability. Compounds X6–X10 demonstrated favorable binding affinities ranging from −7.32 to −7.46 kcal/mol, although these values remained lower than those of the native ligand, estradiol (−10.69 kcal/mol), and tamoxifen (−9.21 kcal/mol). These compounds formed key hydrogen bond interactions with Glu353 and Arg394, similar to estradiol, suggesting a correct binding orientation within the receptor's ligand-binding domain. Structural modifications, particularly the introduction of hydroxyl and fluoro substituents, contributed to the enhanced binding energies observed in these top-performing compounds. ADMET analysis further indicated that compounds X6–X10 complied with Lipinski's Rule of Five, had acceptable oral bioavailability, metabolic stability, and the ability to cross the blood–brain barrier. However, all derivatives were predicted to exhibit mutagenic potential and hepatotoxicity, which may limit their safety profiles. In conclusion, fluoro-hydroxyxanthone derivatives, especially X6–X10, represent promising molecular scaffolds for further optimization and development as potential anti-breast cancer agents targeting ER-α
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.