Main Article Content
Abstract
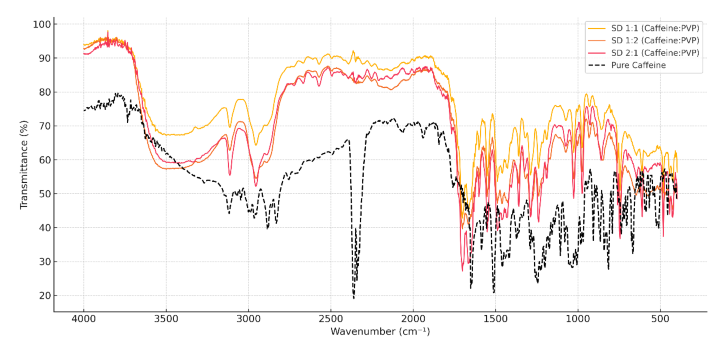
Despite its inherently high aqueous solubility, caffeine demonstrates inconsistent oral bioavailability due to formulation and processing-related limitations. This study aimed to improve the solubility and dissolution rate of caffeine by formulating solid dispersions using polyvinylpyrrolidone K-30 (PVP K-30) via the solvent evaporation method. Solid dispersions were prepared at drug-to-polymer ratios of 1:1, 1:2, and 2:1, and characterized using hot-stage microscopy (HSM), Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), and powder X-ray diffraction (PXRD) analysis. Solubility and dissolution testing were conducted in phosphate buffer (pH 6.8). The results showed that the 1:2 ratio formulation yielded the most significant improvement, with solubility reaching 22.3 mg/mL and a dissolution rate of 97.6% within 30 minutes, representing a substantial enhancement compared to pure caffeine. FTIR and DSC indicated the presence of hydrogen bonding and the absence of caffeine melting peak, while PXRD confirmed amorphization. These findings suggest that solid dispersion with PVP K-30 is a viable strategy for overcoming the bioavailability challenges of caffeine and similar compounds, warranting further investigation into in vivo performance and long-term stability.
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.