Main Article Content
Abstract
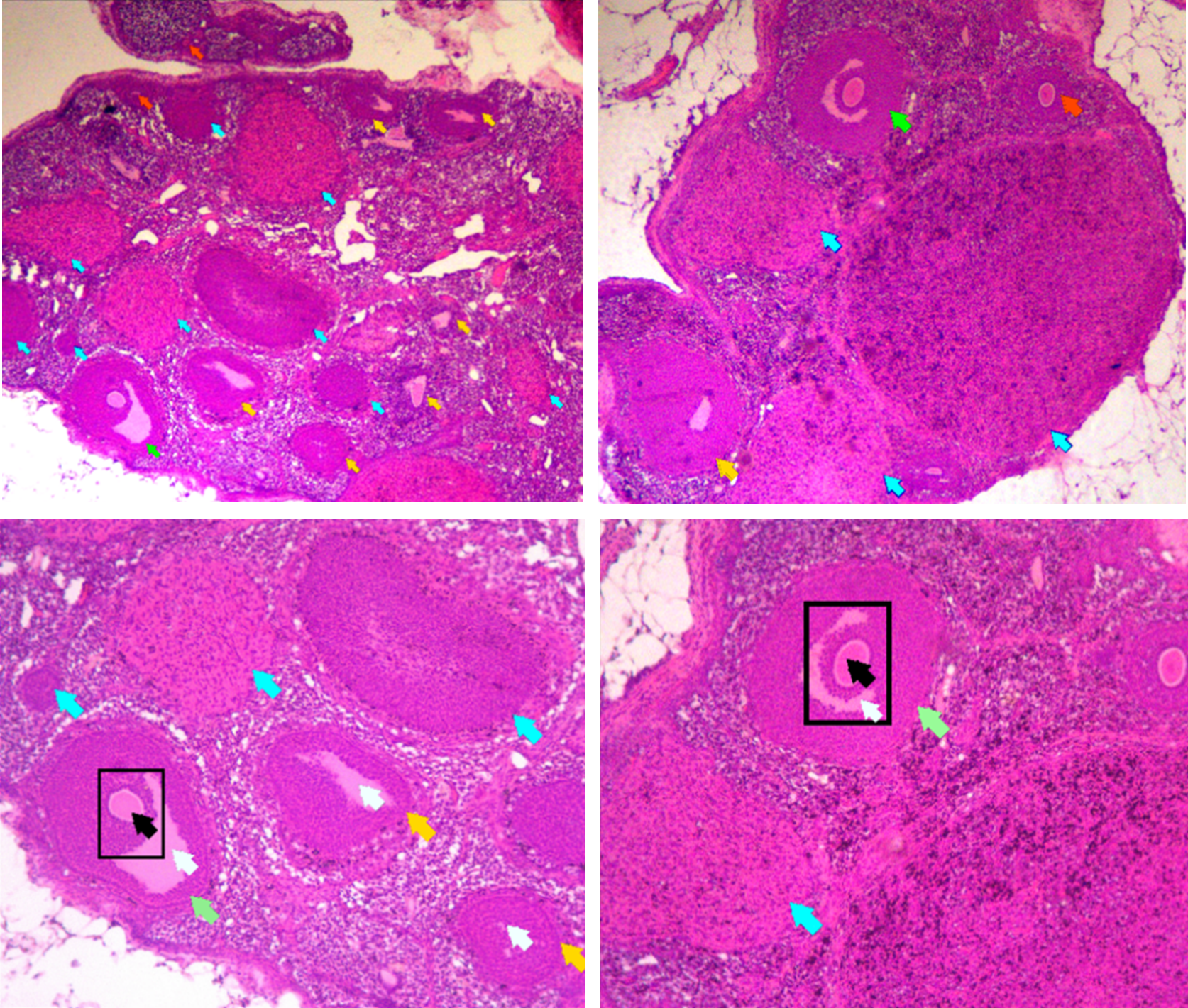
Shallot skin (Allium cepa L.) is a natural material with potential as a traditional medicine due to its high content of the antioxidant quercetin. However, excessive consumption of shallot skin may cause the antioxidant properties of quercetin to shift to pro-oxidant effects via auto-oxidation and metal-binding reactions. Uncontrolled pro-oxidants induce oxidative stress that damages body cells, including ovarian follicle cells. This acute toxicity study aimed to evaluate the toxic effects of shallot skin extract on the ovary using ovarian follicle count as an indicator. This study employed a true experimental design with a posttest-only control group. The method referred to the OECD 420 guideline (fixed dose procedure) using rats (Rattus norvegicus) as test animals. The results showed no significant difference in the mean follicle counts between the control and treatment groups across all follicle categories: primary follicles (p = 0.278), secondary follicles (p = 0.452), DeGraaf follicles (p = 0.39), corpus luteum (p = 0.752), atretic follicles (p = 1.0), and total follicles (p = 0.60). Thus, follicle counts in all categories did not differ significantly (p>0.05). It can be concluded that shallot skin extract does not exert toxic effects on ovarian follicle numbers in rats.
Keywords
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
- Ajayi, A. F. and Akhigbe, R. E. (2020) ‘Staging of the estrous cycle and induction of estrus in experimental rodents: an update’, Fertility Research and Practice, 6(1), pp. 1–15. doi: 10.1186/s40738-020-00074-3.
- Alfiyanti, A., Sitasiwi, A. J. and Mardiati, S. M. (2019) ‘Pengaruh Pemberian Ekstrak Etanol Daun Mimba (Azadirachta indica A.Juss) terhadap Berat Uterus dan Tebal Endometrium Mencit (Mus musculus L.)’, Buletin Anatomi dan Fisiologi, 4(1), pp. 82–89. doi: 10.14710/baf.4.1.2019.82-89.
- ASEAN (2014) “An ASEAN for Evaluating the Guidance Document Safety of Botanical.†ASEAN Botanical Safety Guidance. Available at: https://asean.org/wp-content/uploads/2012/05/ASEAN-Botanical-Safety-Assessment-Guidance.pdf
- BPOM (2022) ‘Peraturan Badan Pengawas Obat Dan Makanan Nomor 10 Tahun 2022 Tentang Pedoman Uji Toksisitas Praklinik Secara In Vivo’, Badan Pengawas Obat dan Makanan Republik Indonesia, pp. 1–220.
- Chen, R. et al. (2014) ‘Potential toxicity of quercetin: The repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow’, Toxicology Reports, 1, pp. 450–458. doi: 10.1016/j.toxrep.2014.07.014.
- Crowe, M. A. (2022) ‘Reproduction, Events and Management: Estrous Cycles: Characteristics’, in McSweeney, P. L. H. and McNamara, J. P. B. T.-E. of D. S. (Third E. (eds). Oxford: Academic Press, pp. 948–953. doi: https://doi.org/10.1016/B978-0-12-818766-1.00079-9.
- DiFiore’s (2008) Atlas of Histology with functional correlations, Vasa. Available at: http://medcontent.metapress.com/index/A65RM03P4874243N.pdf.
- Elsyana, V., & Tutik. (2018) Penapisan Fitokimia dan Skrining Toksisitas Ekstrak Etanol Kulit Bawang Merah. Jurnal Farmasi Malahayati, 1(2), 107–114. Available at: https://ejurnalmalahayati.ac.id/index.php/farmasi/article/view/1543
- Fitriyanti, D., Ulfa, A. M. (2024) Uji Toksisitas ( Brine Shrimp Lethality Test ) terhadap Larva Udang Ekstrak Metano. Kulit Bawang Merah ( Allium Cepa L .) dengan Metode Ekstraksi Sokletasi dan Refluks. Jurnal Farmasi Malahayati, 7(1), 95–104. Available at: https://ejurnalmalahayati.ac.id/index.php/farmasi/article/view/8386
- Garba, S. H. and Dibal, N. I. (2020) ‘Original Article: Acute Toxicity of Quercetin From Onion Skin in Mice’, 6(4), pp. 269–276.
- Kocygit, A. and Selek, S. (2016) ‘Exogenous Antioxidants are Double-edged Swords’, Bezmialem Science, 4(2), pp. 70–75. doi: 10.14235/bs.2016.704.
- Nna, V. U. et al. (2017) ‘Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride - induced oxidative stress in the uterus and ovaries of female Wistar rats’, Food and Chemical Toxicology, 102, pp. 143–155. doi: 10.1016/j.fct.2017.02.010.
- OECD (2016) ‘Test Guideline 421: Reproduction/Developmental Toxicity Screening Test’, OECD Guidelines for the Testing of Chemicals, (421). Available at: http://www.oecd.org/termsandconditions/.
- Putri, A. R. (2020) Efek Gastroprotektif Ekstrak Kulit Bawang Merah Pada Tikus Wistar Jantan Yang Diinduksi Asam Mefenamat, Repository.Unej.Ac.Id. Available at: https://repository.unej.ac.id/handle/123456789/101165%0Ahttps://repository.unej.ac.id/bitstream/handle/123456789/101165/Awalya Rahma Putri 162010101063_1.pdf?sequence=1&isAllowed=y.
- Shahidi, F. and Ambigaipalan, P. (2015) ‘Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects - A review’, Journal of Functional Foods, 18, pp. 820–897. doi: 10.1016/j.jff.2015.06.018.
- Slighoua, M. et al. (2023) ‘Quercetin and Ferulic Acid Elicit Estrogenic Activities In Vivo and In Silico’, Molecules, 28(13), pp. 1–15. doi: 10.3390/molecules28135112.
- Taketa, Y. (2022) ‘Luteal toxicity evaluation in rats’, Journal of Toxicologic Pathology, 35(1), pp. 7–17. doi: 10.1293/tox.2021-0058.
- Tang, Z. R. et al. (2019) ‘Estrogen-receptor expression and function in female reproductive disease’, Cells, 8(10), pp. 1–15. doi: 10.3390/cells8101123.
- Thao, T. H. D., Dung, V. T. N. and Dao, D. Q. (2019) ‘Antioxidant vs. pro-oxidant activities of quercetin in aqueous phase: A Density Functional Theory study’, Vietnam Journal of Chemistry, 57(6), pp. 696–701. doi: 10.1002/vjch.201900085.
References
Ajayi, A. F. and Akhigbe, R. E. (2020) ‘Staging of the estrous cycle and induction of estrus in experimental rodents: an update’, Fertility Research and Practice, 6(1), pp. 1–15. doi: 10.1186/s40738-020-00074-3.
Alfiyanti, A., Sitasiwi, A. J. and Mardiati, S. M. (2019) ‘Pengaruh Pemberian Ekstrak Etanol Daun Mimba (Azadirachta indica A.Juss) terhadap Berat Uterus dan Tebal Endometrium Mencit (Mus musculus L.)’, Buletin Anatomi dan Fisiologi, 4(1), pp. 82–89. doi: 10.14710/baf.4.1.2019.82-89.
ASEAN (2014) “An ASEAN for Evaluating the Guidance Document Safety of Botanical.†ASEAN Botanical Safety Guidance. Available at: https://asean.org/wp-content/uploads/2012/05/ASEAN-Botanical-Safety-Assessment-Guidance.pdf
BPOM (2022) ‘Peraturan Badan Pengawas Obat Dan Makanan Nomor 10 Tahun 2022 Tentang Pedoman Uji Toksisitas Praklinik Secara In Vivo’, Badan Pengawas Obat dan Makanan Republik Indonesia, pp. 1–220.
Chen, R. et al. (2014) ‘Potential toxicity of quercetin: The repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow’, Toxicology Reports, 1, pp. 450–458. doi: 10.1016/j.toxrep.2014.07.014.
Crowe, M. A. (2022) ‘Reproduction, Events and Management: Estrous Cycles: Characteristics’, in McSweeney, P. L. H. and McNamara, J. P. B. T.-E. of D. S. (Third E. (eds). Oxford: Academic Press, pp. 948–953. doi: https://doi.org/10.1016/B978-0-12-818766-1.00079-9.
DiFiore’s (2008) Atlas of Histology with functional correlations, Vasa. Available at: http://medcontent.metapress.com/index/A65RM03P4874243N.pdf.
Elsyana, V., & Tutik. (2018) Penapisan Fitokimia dan Skrining Toksisitas Ekstrak Etanol Kulit Bawang Merah. Jurnal Farmasi Malahayati, 1(2), 107–114. Available at: https://ejurnalmalahayati.ac.id/index.php/farmasi/article/view/1543
Fitriyanti, D., Ulfa, A. M. (2024) Uji Toksisitas ( Brine Shrimp Lethality Test ) terhadap Larva Udang Ekstrak Metano. Kulit Bawang Merah ( Allium Cepa L .) dengan Metode Ekstraksi Sokletasi dan Refluks. Jurnal Farmasi Malahayati, 7(1), 95–104. Available at: https://ejurnalmalahayati.ac.id/index.php/farmasi/article/view/8386
Garba, S. H. and Dibal, N. I. (2020) ‘Original Article: Acute Toxicity of Quercetin From Onion Skin in Mice’, 6(4), pp. 269–276.
Kocygit, A. and Selek, S. (2016) ‘Exogenous Antioxidants are Double-edged Swords’, Bezmialem Science, 4(2), pp. 70–75. doi: 10.14235/bs.2016.704.
Nna, V. U. et al. (2017) ‘Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride - induced oxidative stress in the uterus and ovaries of female Wistar rats’, Food and Chemical Toxicology, 102, pp. 143–155. doi: 10.1016/j.fct.2017.02.010.
OECD (2016) ‘Test Guideline 421: Reproduction/Developmental Toxicity Screening Test’, OECD Guidelines for the Testing of Chemicals, (421). Available at: http://www.oecd.org/termsandconditions/.
Putri, A. R. (2020) Efek Gastroprotektif Ekstrak Kulit Bawang Merah Pada Tikus Wistar Jantan Yang Diinduksi Asam Mefenamat, Repository.Unej.Ac.Id. Available at: https://repository.unej.ac.id/handle/123456789/101165%0Ahttps://repository.unej.ac.id/bitstream/handle/123456789/101165/Awalya Rahma Putri 162010101063_1.pdf?sequence=1&isAllowed=y.
Shahidi, F. and Ambigaipalan, P. (2015) ‘Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects - A review’, Journal of Functional Foods, 18, pp. 820–897. doi: 10.1016/j.jff.2015.06.018.
Slighoua, M. et al. (2023) ‘Quercetin and Ferulic Acid Elicit Estrogenic Activities In Vivo and In Silico’, Molecules, 28(13), pp. 1–15. doi: 10.3390/molecules28135112.
Taketa, Y. (2022) ‘Luteal toxicity evaluation in rats’, Journal of Toxicologic Pathology, 35(1), pp. 7–17. doi: 10.1293/tox.2021-0058.
Tang, Z. R. et al. (2019) ‘Estrogen-receptor expression and function in female reproductive disease’, Cells, 8(10), pp. 1–15. doi: 10.3390/cells8101123.
Thao, T. H. D., Dung, V. T. N. and Dao, D. Q. (2019) ‘Antioxidant vs. pro-oxidant activities of quercetin in aqueous phase: A Density Functional Theory study’, Vietnam Journal of Chemistry, 57(6), pp. 696–701. doi: 10.1002/vjch.201900085.