Main Article Content
Abstract
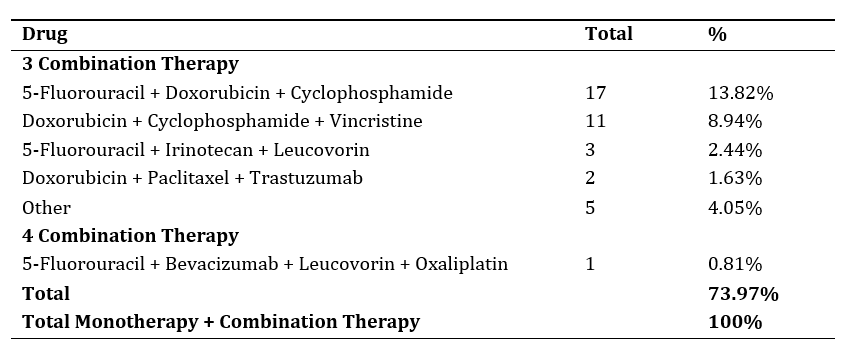
Chemotherapy is commonly used in cancer patients either as a monotherapy or in combination, as it demonstrates higher effectiveness and lower toxicity compared to single-agent use, while also preventing drug resistance. The combination of chemotherapy drugs, or their use alongside supportive drugs, can increase the risk of drug interactions that may affect treatment outcomes. The purpose of this research is to examine and offer suggestions for the management of medication interactions in cancer patients at the X Cancer Centre polyclinic of X Denpasar Hospital in 2020. The present investigation is a cross-sectional descriptive study with retrospective data collection from medical records in 2020. Drug interaction data were analyzed using Drugs.com, Lexicomp, and Stockley to assess the type of interaction, risk level, severity, and management of each interaction. The results indicated that the most common types of cancer were breast cancer (62.7%) and lymphoma (10.2%), with combination chemotherapy being used in 73.97% of cases. The most frequent type of interaction was pharmacodynamic interaction (50.42%), with risk level C (35.53%) and moderate severity (69.07%). The most common interactions were between chemotherapy drugs and supportive drugs (46.47%). The recommended management of potential drug interactions in cancer patients includes providing a time gap between drug administrations.
Keywords
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
References
- Alipour, M. (2021), “Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancerâ€, Journal of Gastrointestinal Cancer, Vol. 52, pp. 23–30, doi: 10.1007/s12029-020-00518-5/Published.
- Amtiria, H.R., Khairun, D. and Berawi, N. (2018), Peran Human Epidermal Growth Factor Receptor-2 Pada Kanker Payudara, J Agromedicine Unila |, Vol. 5.
- Atalay, F., Gulmez, O. and Ozsancak Ugurlu, A. (2014), “Cardiotoxicity following cyclophosphamide therapy: a case reportâ€, Journal of Medical Case Reports, BioMed Central Ltd., Vol. 8 No. 1, doi: 10.1186/1752-1947-8-252.
- Büyükköroǧlu, G., Åženel, B., Gezgin, S. and Dinh, T. (2016), “The simultaneous delivery of paclitaxel and Herceptin® using solid lipid nanoparticles: In vitro evaluationâ€, Journal of Drug Delivery Science and Technology, Editions de Sante, Vol. 35, pp. 98–105, doi: 10.1016/j.jddst.2016.06.010.
- D’Errico, S., Baldari, B., Arcangeli, M., Santurro, A., Frati, P. and Fineschi, V. (2020), “Mast cells activation and high blood tryptase levels due to paclitaxel administration. Is Cremophor EL the culprit? A case reportâ€, Medicine (United States), Lippincott Williams and Wilkins, Vol. 99 No. 43, doi: 10.1097/MD.0000000000022814.
- Drug.com. (2023), “Drug Interaction Checker: https://www.drugs.com/drug_interactions.htmlâ€.
- Elmika, E. and Adi, M.S.A. (2020), “Gambaran Umur, dan Jenis Kelamin Pasien Kanker Payudara di RS Ibnu Sina Kota Makassarâ€, Jurnal Penelitian Kesehatan “SUARA FORIKES†(Journal of Health Research “Forikes Voiceâ€), Forum Ilmiah Kesehatan - Forikes, Vol. 11 No. 1, p. 1, doi: 10.33846/sf11101.
- Faizah, A.K. (2018), “Kajian Interaksi Obat Pada Pasien Pneumonia Di RS Pendidikan Surabayaâ€, SCIENTIA Jurnal Farmasi Dan Kesehatan, Vol. 8 No. 1, pp. 86–91.
- Feinstein, J., Dai, D., Zhong, W., Freedman, J. and Feudtner, C. (2015), “Potential drug-drug interactions in infant, child, and adolescent patients in Children’s Hospitalsâ€, Pediatrics, American Academy of Pediatrics, Vol. 135 No. 1, pp. e99–e108, doi: 10.1542/peds.2014-2015.
- Firdaus, N.Z. and Susilowati, S. (2023), “Evaluasi Penggunaan Kemoterapi Pada Pasien Kanker Payudara Di Rumah Sakit Islam Sultan Agung Semarang Tahun 2022â€, Jurnal Ilmu Farmasi Dan Farmasi Klinik (JIFFK), Vol. 20 No. 2, pp. 155–166.
- Fritz, I., Wagner, P. and Olsson, H. (2021), “Improved survival in several cancers with use of H1-antihistamines desloratadine and loratadineâ€, Translational Oncology, Neoplasia Press, Inc., Vol. 14 No. 4, doi: 10.1016/j.tranon.2021.101029.
- Hammad, M.A., Tangiisuran, B., Kharshid, A.M., Abdul-Aziz, N., Hassan, Y., Aziz, N.A. and Elsayed, T.M. (2017), “Drug-drug interaction-related uncontrolled glycemiaâ€, Journal of Pharmacy and Bioallied Sciences, Medknow Publications, Vol. 9 No. 4, pp. 221–228, doi: 10.4103/JPBS.JPBS_26_17.
- Hasnita, Y. and Arif Harahap, W. (2019), Pengaruh Faktor Risiko Hormonal Pada Pasien Kanker Payudara Di RSUP.Dr.M.Djamil Padang, Jurnal Kesehatan Andalas, Vol. 8.
- Irawati and Sardjan, M. (2022), “Pola Peresepan Obat Kemoterapi Kanker Payudara di Rumah Sakit Lavalette Kota Malangâ€, PHARMADEMICA : Jurnal Kefarmasian Dan Gizi, LPPM-KI Akademi Analis Farmasi dan Makanan & Akademi Farmasi Putra Indonesia Malang, Vol. 1 No. 2, pp. 80–85, doi: 10.54445/pharmademica.v1i2.12.
- Ismail, M., Khan, S., Khan, F., Noor, S., Sajid, H., Yar, S. and Rasheed, I. (2020), “Prevalence and significance of potential drug-drug interactions among cancer patients receiving chemotherapyâ€, BMC Cancer, BioMed Central Ltd., Vol. 20 No. 1, doi: 10.1186/s12885-020-06855-9.
- Jamali, J., Dayo, A., Memon, N., Mughal, U.-R., Khatri, M.A., Muhammad, S., Qureshi, Y., et al. (2021), “Assessment of Patient’s Response about Adverse Drug Reactions Receiving AC (Adriamycin, Cyclophosphamide) Therapy: A Survey Researchâ€, Journal of Pharmaceutical Research International, Sciencedomain International, pp. 8–13, doi: 10.9734/jpri/2021/v33i26a31467.
- Koni, A.A., Nazzal, M.A., Suwan, B.A., Sobuh, S.S., Abuhazeem, N.T., Salman, A.N., Salameh, H.T., et al. (2022), “A comprehensive evaluation of potentially significant drug-drug, drug-herb, and drug-food interactions among cancer patients receiving anticancer drugsâ€, BMC Cancer, BioMed Central Ltd, Vol. 22 No. 1, doi: 10.1186/s12885-022-09649-3.
- Kurniawati, F., Yasin, N.M., Dina, A., Atana, S. and Hakim, S.N. (2021), “Kajian Adverse Drug Reactions Terkait Interaksi Obat di Bangsal Rawat Inap Rumah Sakit Akademik UGMâ€, JURNAL MANAJEMEN DAN PELAYANAN FARMASI (Journal of Management and Pharmacy Practice), Universitas Gadjah Mada, Vol. 10 No. 4, doi: 10.22146/jmpf.60228.
- Laban, A., Birand, N., Chukwunyere, U., Abdi, A. and Basgut, B. (2021), “Evaluation of drug-drug interactions in cancer patients treated at a university hospital in North Cyprus using two interaction databasesâ€, Nigerian Journal of Clinical Practice, Wolters Kluwer Medknow Publications, Vol. 24 No. 7, pp. 1067–1071, doi: 10.4103/njcp.njcp_266_20.
- de Lemos, M.L., Kung, C., Kletas, V., Badry, N. and Kang, I. (2019), “Approach to initiating QT-prolonging oncology drugs in the ambulatory settingâ€, Journal of Oncology Pharmacy Practice, SAGE Publications Ltd, 1 January, doi: 10.1177/1078155217748735.
- Lexicom. (2023), “Drug Referential Solution: https://www.wolterskluwer.com/en/solutions/uptodate/enterprise/lexidrugâ€.
- Mantang, A., Useng, Y. and Pusmarani, J. (2023), “Hubungan Drug Related Problems (DRP) Kategori Interaksi Obat Pada Penggunaan Obat Pasien Hipertensi di Puskesmas Lalonggasumeeto Kabupaten Konaweâ€, Jurnal Pharmacia Mandala Waluya, Lembaga Penelitian dan Pengabdian Masyarakat Universitas Mandala Waluya, Vol. 2 No. 5, pp. 286–294, doi: 10.54883/jpmw.v2i5.60.
- Nayak, J., Sahoo, S.K. and Kumar, R. (2021), “Study of Anticancer Drugs Interaction with Hemoglobin by Electrochemical Methods and Molecular Docking: Implications towards Anticancer Treatmentâ€, ChemistrySelect, John Wiley and Sons Inc, Vol. 6 No. 17, pp. 4098–4106, doi: 10.1002/slct.202100424.
- Novita, N.F. and Destiani, D.P. (2019), “Interkasi Obat Terhadap Perpanjangan Interval Qtâ€, Farmaka, Vol. 18 No. 1, pp. 110–118.
- Pereira-Oliveira, M., Reis-Mendes, A., Carvalho, F., Remião, F., Bastos, M. de L. and Costa, V.M. (2019), “Doxorubicin is key for the cardiotoxicity of FAC (5-fluorouracil + adriamycin + cyclophosphamide) combination in differentiated H9c2 cellsâ€, Biomolecules, MDPI AG, Vol. 9 No. 1, doi: 10.3390/biom9010021.
- Permana, K.A.W., Agung Yudistira Permana, M., Nisa, S., Kedokteran, F., Lampung, U., Ba, R. and Encik Maryam, R. (2019), “Asosiasi Triple Negative Breast Cancer (TNBC) Dengan Mutasi BRCA-1 Dan Etnisitasâ€, Medula, Vol. 9 No. 3, p. 398.
- Rabba, A.K., Abu Hussein, A.M., Bayan K, Sbeih, A. and Nasser, S.I. (2020), “Assessing Drug-Drug Interaction Potential among Patients Admitted to Surgery Departments in Three Palestinian Hospitalsâ€, BioMed Research International, pp. 1–6.
- Ramasubbu, S.K., Mahato, S.K., Agnihotri, A., Pasricha, R.K., Nath, U.K. and Das, B. (2021), “Prevalence, severity, and nature of risk factors associated with drug-drug interactions in geriatric patients receiving cancer chemotherapy: A prospective study in a tertiary care teaching hospitalâ€, Cancer Treatment and Research Communications, Elsevier Ltd, Vol. 26, doi: 10.1016/j.ctarc.2020.100277.
- Ramdani, R., Skarayadi, O., Indrawati, W., Hermanto, F., Wahyuni, E., Jenderal Achmad Yani, U., Farmasi, I., et al. (2022), Potensi Interaksi Obat Antihipertensi Pada Pasien Geriatri Rawat Inap Di Salah Satu Rumah Sakit Kota Bandung, Potensi Interaksi Obat Antihipertensi…..Pharmacoscript, Vol. 5.
- Riechelmann, R.P. and Krzyzanowska, M.K. (2019), “Drug interactions and oncological outcomes: A hidden adversaryâ€, Ecancermedicalscience, ecancer Global Foundation, doi: 10.3332/ECANCER.2019.ED88.
- Rizo, L.M., Sánchez Gómez-Serranillos, M., Cantero, M.D., Carlos, L., Lisón, F., Peinado, I.I., Pilar, M., et al. (2020), “Drug-Drug Interaction of Oral Antineoplastic Agents in the Cancer Patientâ€, Journal of Clinical Oncology, Vol. 2 No. 1, pp. 1–7.
- Rusdi, N.K., Sari, E.N. and Wulandari, N. (2023), “Ketepatan Obat, Dosis, dan Potensi Interaksi Obat pada Pasien Kanker Paru di Rumah Sakit X Jawa Barat Periode 2019-2021â€, Jurnal Sains Dan Kesehatan, Faculty of Pharmacy, Mulawarman University, Vol. 5 No. 3, pp. 313–323, doi: 10.25026/jsk.v5i3.1754.
- Sari, D.P. and Gumayesty, Y. (2016), “Faktor-Faktor Yang Berhubungan Dengan Kejadian Kanker Payudara Di Poliklinik Onkologi Rsud Arifin Achmad Provinsi Riauâ€, Jurnal Ilmu Kesehatan Masyarakat, STIKES Al- Insyirah Pekanbaru, Vol. 05 No. 02, pp. 84–92.
- Shetty, V., Chowta, M.N., Chowta K, N., Shenoy, A., Kamath, A. and Kamath, P. (2018), “Evaluation of Potential Drug-Drug Interactions with Medications Prescribed to Geriatric Patients in a Tertiary Care Hospitalâ€, Journal of Aging Research, Hindawi Limited, Vol. 2018, doi: 10.1155/2018/5728957.
- Shinta R, N. and Surarso, B. (2016), “Terapi Mual dan Muntah Pasca Kemoterapiâ€, Jurnal THT-KL, Vol. 9 No. 2, pp. 74–83.
- Sun, D., Li, H., Cao, M., He, S., Lei, L., Peng, J. and Chen, W. (2020), “Cancer burden in China: trends, risk factors and preventionâ€, Cancer Biology and Medicine, Cancer Biology and Medicine, 1 November, doi: 10.20892/j.issn.2095-3941.2020.0387.
- Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A. and Bray, F. (2021), “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countriesâ€, CA: A Cancer Journal for Clinicians, Wiley, Vol. 71 No. 3, pp. 209–249, doi: 10.3322/caac.21660.
- Umar, R.M. (2018), “Drug-drug interactions between antiemetics used in cancer patientsâ€, Journal of Oncological Sciences, Journal of Oncological Sciences (JOS), Vol. 4 No. 3, pp. 142–146, doi: 10.1016/j.jons.2018.07.003.
- Wardana, N. and Ernawati, R. (2019), “Hubungan Usia dan Aktivitas Fisik dengan Jenis Kanker di Ruang Kemoterapi RSUD Abdul Wahab Sjahranie Samarindaâ€, Borneo Student Research, pp. 159–165.
- Williamson, S. and Polwart, C. (2016), “Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelinesâ€, pp. 1–16.
- Wu, R., Zhang, Z., Wang, B., Chen, G., Zhang, Y., Deng, H., Tang, Z., et al. (2020), “Combination chemotherapy of lung cancer – co-delivery of docetaxel prodrug and cisplatin using aptamer-decorated lipid–polymer hybrid nanoparticlesâ€, Drug Design, Development and Therapy, Dove Medical Press Ltd., Vol. 14, pp. 2249–2261, doi: 10.2147/DDDT.S246574.
- Yeoh, T.T., Tay, X.Y., Si, P. and Chew, L. (2015), “Drug-related problems in elderly patients with cancer receiving outpatient chemotherapyâ€, Journal of Geriatric Oncology, Elsevier Ltd, Vol. 6 No. 4, pp. 280–287, doi: 10.1016/j.jgo.2015.05.001.
- Yu, K. Da, Ye, F.G., He, M., Fan, L., Ma, D., Mo, M., Wu, J., et al. (2020), “Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women with Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trialâ€, JAMA Oncology, American Medical Association, Vol. 6 No. 9, pp. 1390–1396, doi: 10.1001/jamaoncol.2020.2965.
- Yuliawati, Dhigna, Sardiana, E. and Savira, R.D. (2021), “Analisis Potensi Interaksi Obat Pasien Geriatri di Bangsal Penyakit Dalam Salah Satu Rumah Sakit di Jambi Analysis of Drug Interaction Potential among Geriatric in the Internal Medicine Inpatient Ward at One of Hospital in Jambiâ€, Indonesian Journal of Pharma Science, Vol. 1 No. 1, pp. 21–27.
References
Alipour, M. (2021), “Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancerâ€, Journal of Gastrointestinal Cancer, Vol. 52, pp. 23–30, doi: 10.1007/s12029-020-00518-5/Published.
Amtiria, H.R., Khairun, D. and Berawi, N. (2018), Peran Human Epidermal Growth Factor Receptor-2 Pada Kanker Payudara, J Agromedicine Unila |, Vol. 5.
Atalay, F., Gulmez, O. and Ozsancak Ugurlu, A. (2014), “Cardiotoxicity following cyclophosphamide therapy: a case reportâ€, Journal of Medical Case Reports, BioMed Central Ltd., Vol. 8 No. 1, doi: 10.1186/1752-1947-8-252.
Büyükköroǧlu, G., Åženel, B., Gezgin, S. and Dinh, T. (2016), “The simultaneous delivery of paclitaxel and Herceptin® using solid lipid nanoparticles: In vitro evaluationâ€, Journal of Drug Delivery Science and Technology, Editions de Sante, Vol. 35, pp. 98–105, doi: 10.1016/j.jddst.2016.06.010.
D’Errico, S., Baldari, B., Arcangeli, M., Santurro, A., Frati, P. and Fineschi, V. (2020), “Mast cells activation and high blood tryptase levels due to paclitaxel administration. Is Cremophor EL the culprit? A case reportâ€, Medicine (United States), Lippincott Williams and Wilkins, Vol. 99 No. 43, doi: 10.1097/MD.0000000000022814.
Drug.com. (2023), “Drug Interaction Checker: https://www.drugs.com/drug_interactions.htmlâ€.
Elmika, E. and Adi, M.S.A. (2020), “Gambaran Umur, dan Jenis Kelamin Pasien Kanker Payudara di RS Ibnu Sina Kota Makassarâ€, Jurnal Penelitian Kesehatan “SUARA FORIKES†(Journal of Health Research “Forikes Voiceâ€), Forum Ilmiah Kesehatan - Forikes, Vol. 11 No. 1, p. 1, doi: 10.33846/sf11101.
Faizah, A.K. (2018), “Kajian Interaksi Obat Pada Pasien Pneumonia Di RS Pendidikan Surabayaâ€, SCIENTIA Jurnal Farmasi Dan Kesehatan, Vol. 8 No. 1, pp. 86–91.
Feinstein, J., Dai, D., Zhong, W., Freedman, J. and Feudtner, C. (2015), “Potential drug-drug interactions in infant, child, and adolescent patients in Children’s Hospitalsâ€, Pediatrics, American Academy of Pediatrics, Vol. 135 No. 1, pp. e99–e108, doi: 10.1542/peds.2014-2015.
Firdaus, N.Z. and Susilowati, S. (2023), “Evaluasi Penggunaan Kemoterapi Pada Pasien Kanker Payudara Di Rumah Sakit Islam Sultan Agung Semarang Tahun 2022â€, Jurnal Ilmu Farmasi Dan Farmasi Klinik (JIFFK), Vol. 20 No. 2, pp. 155–166.
Fritz, I., Wagner, P. and Olsson, H. (2021), “Improved survival in several cancers with use of H1-antihistamines desloratadine and loratadineâ€, Translational Oncology, Neoplasia Press, Inc., Vol. 14 No. 4, doi: 10.1016/j.tranon.2021.101029.
Hammad, M.A., Tangiisuran, B., Kharshid, A.M., Abdul-Aziz, N., Hassan, Y., Aziz, N.A. and Elsayed, T.M. (2017), “Drug-drug interaction-related uncontrolled glycemiaâ€, Journal of Pharmacy and Bioallied Sciences, Medknow Publications, Vol. 9 No. 4, pp. 221–228, doi: 10.4103/JPBS.JPBS_26_17.
Hasnita, Y. and Arif Harahap, W. (2019), Pengaruh Faktor Risiko Hormonal Pada Pasien Kanker Payudara Di RSUP.Dr.M.Djamil Padang, Jurnal Kesehatan Andalas, Vol. 8.
Irawati and Sardjan, M. (2022), “Pola Peresepan Obat Kemoterapi Kanker Payudara di Rumah Sakit Lavalette Kota Malangâ€, PHARMADEMICA : Jurnal Kefarmasian Dan Gizi, LPPM-KI Akademi Analis Farmasi dan Makanan & Akademi Farmasi Putra Indonesia Malang, Vol. 1 No. 2, pp. 80–85, doi: 10.54445/pharmademica.v1i2.12.
Ismail, M., Khan, S., Khan, F., Noor, S., Sajid, H., Yar, S. and Rasheed, I. (2020), “Prevalence and significance of potential drug-drug interactions among cancer patients receiving chemotherapyâ€, BMC Cancer, BioMed Central Ltd., Vol. 20 No. 1, doi: 10.1186/s12885-020-06855-9.
Jamali, J., Dayo, A., Memon, N., Mughal, U.-R., Khatri, M.A., Muhammad, S., Qureshi, Y., et al. (2021), “Assessment of Patient’s Response about Adverse Drug Reactions Receiving AC (Adriamycin, Cyclophosphamide) Therapy: A Survey Researchâ€, Journal of Pharmaceutical Research International, Sciencedomain International, pp. 8–13, doi: 10.9734/jpri/2021/v33i26a31467.
Koni, A.A., Nazzal, M.A., Suwan, B.A., Sobuh, S.S., Abuhazeem, N.T., Salman, A.N., Salameh, H.T., et al. (2022), “A comprehensive evaluation of potentially significant drug-drug, drug-herb, and drug-food interactions among cancer patients receiving anticancer drugsâ€, BMC Cancer, BioMed Central Ltd, Vol. 22 No. 1, doi: 10.1186/s12885-022-09649-3.
Kurniawati, F., Yasin, N.M., Dina, A., Atana, S. and Hakim, S.N. (2021), “Kajian Adverse Drug Reactions Terkait Interaksi Obat di Bangsal Rawat Inap Rumah Sakit Akademik UGMâ€, JURNAL MANAJEMEN DAN PELAYANAN FARMASI (Journal of Management and Pharmacy Practice), Universitas Gadjah Mada, Vol. 10 No. 4, doi: 10.22146/jmpf.60228.
Laban, A., Birand, N., Chukwunyere, U., Abdi, A. and Basgut, B. (2021), “Evaluation of drug-drug interactions in cancer patients treated at a university hospital in North Cyprus using two interaction databasesâ€, Nigerian Journal of Clinical Practice, Wolters Kluwer Medknow Publications, Vol. 24 No. 7, pp. 1067–1071, doi: 10.4103/njcp.njcp_266_20.
de Lemos, M.L., Kung, C., Kletas, V., Badry, N. and Kang, I. (2019), “Approach to initiating QT-prolonging oncology drugs in the ambulatory settingâ€, Journal of Oncology Pharmacy Practice, SAGE Publications Ltd, 1 January, doi: 10.1177/1078155217748735.
Lexicom. (2023), “Drug Referential Solution: https://www.wolterskluwer.com/en/solutions/uptodate/enterprise/lexidrugâ€.
Mantang, A., Useng, Y. and Pusmarani, J. (2023), “Hubungan Drug Related Problems (DRP) Kategori Interaksi Obat Pada Penggunaan Obat Pasien Hipertensi di Puskesmas Lalonggasumeeto Kabupaten Konaweâ€, Jurnal Pharmacia Mandala Waluya, Lembaga Penelitian dan Pengabdian Masyarakat Universitas Mandala Waluya, Vol. 2 No. 5, pp. 286–294, doi: 10.54883/jpmw.v2i5.60.
Nayak, J., Sahoo, S.K. and Kumar, R. (2021), “Study of Anticancer Drugs Interaction with Hemoglobin by Electrochemical Methods and Molecular Docking: Implications towards Anticancer Treatmentâ€, ChemistrySelect, John Wiley and Sons Inc, Vol. 6 No. 17, pp. 4098–4106, doi: 10.1002/slct.202100424.
Novita, N.F. and Destiani, D.P. (2019), “Interkasi Obat Terhadap Perpanjangan Interval Qtâ€, Farmaka, Vol. 18 No. 1, pp. 110–118.
Pereira-Oliveira, M., Reis-Mendes, A., Carvalho, F., Remião, F., Bastos, M. de L. and Costa, V.M. (2019), “Doxorubicin is key for the cardiotoxicity of FAC (5-fluorouracil + adriamycin + cyclophosphamide) combination in differentiated H9c2 cellsâ€, Biomolecules, MDPI AG, Vol. 9 No. 1, doi: 10.3390/biom9010021.
Permana, K.A.W., Agung Yudistira Permana, M., Nisa, S., Kedokteran, F., Lampung, U., Ba, R. and Encik Maryam, R. (2019), “Asosiasi Triple Negative Breast Cancer (TNBC) Dengan Mutasi BRCA-1 Dan Etnisitasâ€, Medula, Vol. 9 No. 3, p. 398.
Rabba, A.K., Abu Hussein, A.M., Bayan K, Sbeih, A. and Nasser, S.I. (2020), “Assessing Drug-Drug Interaction Potential among Patients Admitted to Surgery Departments in Three Palestinian Hospitalsâ€, BioMed Research International, pp. 1–6.
Ramasubbu, S.K., Mahato, S.K., Agnihotri, A., Pasricha, R.K., Nath, U.K. and Das, B. (2021), “Prevalence, severity, and nature of risk factors associated with drug-drug interactions in geriatric patients receiving cancer chemotherapy: A prospective study in a tertiary care teaching hospitalâ€, Cancer Treatment and Research Communications, Elsevier Ltd, Vol. 26, doi: 10.1016/j.ctarc.2020.100277.
Ramdani, R., Skarayadi, O., Indrawati, W., Hermanto, F., Wahyuni, E., Jenderal Achmad Yani, U., Farmasi, I., et al. (2022), Potensi Interaksi Obat Antihipertensi Pada Pasien Geriatri Rawat Inap Di Salah Satu Rumah Sakit Kota Bandung, Potensi Interaksi Obat Antihipertensi…..Pharmacoscript, Vol. 5.
Riechelmann, R.P. and Krzyzanowska, M.K. (2019), “Drug interactions and oncological outcomes: A hidden adversaryâ€, Ecancermedicalscience, ecancer Global Foundation, doi: 10.3332/ECANCER.2019.ED88.
Rizo, L.M., Sánchez Gómez-Serranillos, M., Cantero, M.D., Carlos, L., Lisón, F., Peinado, I.I., Pilar, M., et al. (2020), “Drug-Drug Interaction of Oral Antineoplastic Agents in the Cancer Patientâ€, Journal of Clinical Oncology, Vol. 2 No. 1, pp. 1–7.
Rusdi, N.K., Sari, E.N. and Wulandari, N. (2023), “Ketepatan Obat, Dosis, dan Potensi Interaksi Obat pada Pasien Kanker Paru di Rumah Sakit X Jawa Barat Periode 2019-2021â€, Jurnal Sains Dan Kesehatan, Faculty of Pharmacy, Mulawarman University, Vol. 5 No. 3, pp. 313–323, doi: 10.25026/jsk.v5i3.1754.
Sari, D.P. and Gumayesty, Y. (2016), “Faktor-Faktor Yang Berhubungan Dengan Kejadian Kanker Payudara Di Poliklinik Onkologi Rsud Arifin Achmad Provinsi Riauâ€, Jurnal Ilmu Kesehatan Masyarakat, STIKES Al- Insyirah Pekanbaru, Vol. 05 No. 02, pp. 84–92.
Shetty, V., Chowta, M.N., Chowta K, N., Shenoy, A., Kamath, A. and Kamath, P. (2018), “Evaluation of Potential Drug-Drug Interactions with Medications Prescribed to Geriatric Patients in a Tertiary Care Hospitalâ€, Journal of Aging Research, Hindawi Limited, Vol. 2018, doi: 10.1155/2018/5728957.
Shinta R, N. and Surarso, B. (2016), “Terapi Mual dan Muntah Pasca Kemoterapiâ€, Jurnal THT-KL, Vol. 9 No. 2, pp. 74–83.
Sun, D., Li, H., Cao, M., He, S., Lei, L., Peng, J. and Chen, W. (2020), “Cancer burden in China: trends, risk factors and preventionâ€, Cancer Biology and Medicine, Cancer Biology and Medicine, 1 November, doi: 10.20892/j.issn.2095-3941.2020.0387.
Sung, H., Ferlay, J., Siegel, R.L., Laversanne, M., Soerjomataram, I., Jemal, A. and Bray, F. (2021), “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countriesâ€, CA: A Cancer Journal for Clinicians, Wiley, Vol. 71 No. 3, pp. 209–249, doi: 10.3322/caac.21660.
Umar, R.M. (2018), “Drug-drug interactions between antiemetics used in cancer patientsâ€, Journal of Oncological Sciences, Journal of Oncological Sciences (JOS), Vol. 4 No. 3, pp. 142–146, doi: 10.1016/j.jons.2018.07.003.
Wardana, N. and Ernawati, R. (2019), “Hubungan Usia dan Aktivitas Fisik dengan Jenis Kanker di Ruang Kemoterapi RSUD Abdul Wahab Sjahranie Samarindaâ€, Borneo Student Research, pp. 159–165.
Williamson, S. and Polwart, C. (2016), “Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelinesâ€, pp. 1–16.
Wu, R., Zhang, Z., Wang, B., Chen, G., Zhang, Y., Deng, H., Tang, Z., et al. (2020), “Combination chemotherapy of lung cancer – co-delivery of docetaxel prodrug and cisplatin using aptamer-decorated lipid–polymer hybrid nanoparticlesâ€, Drug Design, Development and Therapy, Dove Medical Press Ltd., Vol. 14, pp. 2249–2261, doi: 10.2147/DDDT.S246574.
Yeoh, T.T., Tay, X.Y., Si, P. and Chew, L. (2015), “Drug-related problems in elderly patients with cancer receiving outpatient chemotherapyâ€, Journal of Geriatric Oncology, Elsevier Ltd, Vol. 6 No. 4, pp. 280–287, doi: 10.1016/j.jgo.2015.05.001.
Yu, K. Da, Ye, F.G., He, M., Fan, L., Ma, D., Mo, M., Wu, J., et al. (2020), “Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women with Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trialâ€, JAMA Oncology, American Medical Association, Vol. 6 No. 9, pp. 1390–1396, doi: 10.1001/jamaoncol.2020.2965.
Yuliawati, Dhigna, Sardiana, E. and Savira, R.D. (2021), “Analisis Potensi Interaksi Obat Pasien Geriatri di Bangsal Penyakit Dalam Salah Satu Rumah Sakit di Jambi Analysis of Drug Interaction Potential among Geriatric in the Internal Medicine Inpatient Ward at One of Hospital in Jambiâ€, Indonesian Journal of Pharma Science, Vol. 1 No. 1, pp. 21–27.